
Electron Energy Level Equations & Examples | What is an Energy Level of an Atom? - Video & Lesson Transcript | Study.com
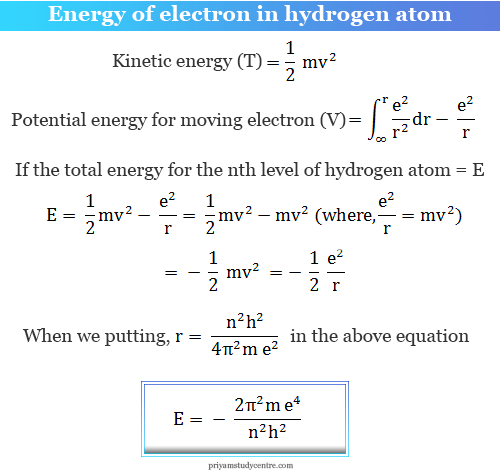
Using Bohr's formula for energy quantization, the ionisation potential of first excited state of hydrogen atom is: . (1) 13.6V, (2) 3.4V, (3) 2.6V, (4) 1.51V

Calculate the energy required to excite an electron in hydrogen atom from the ground state to - YouTube

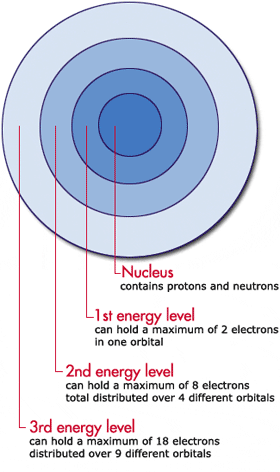
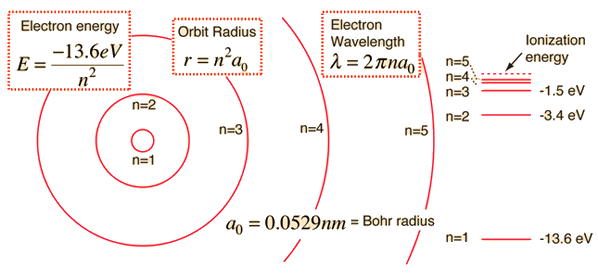





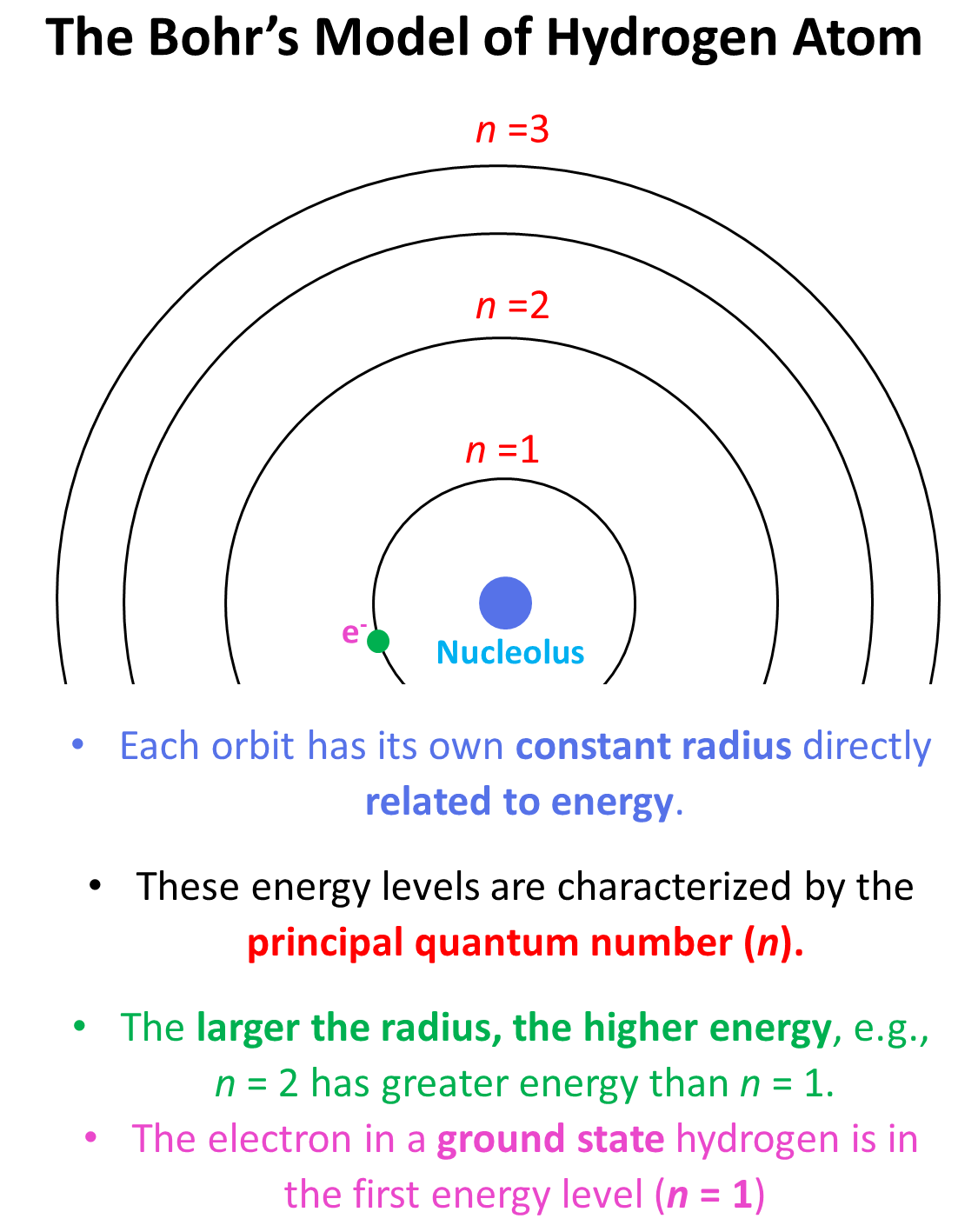
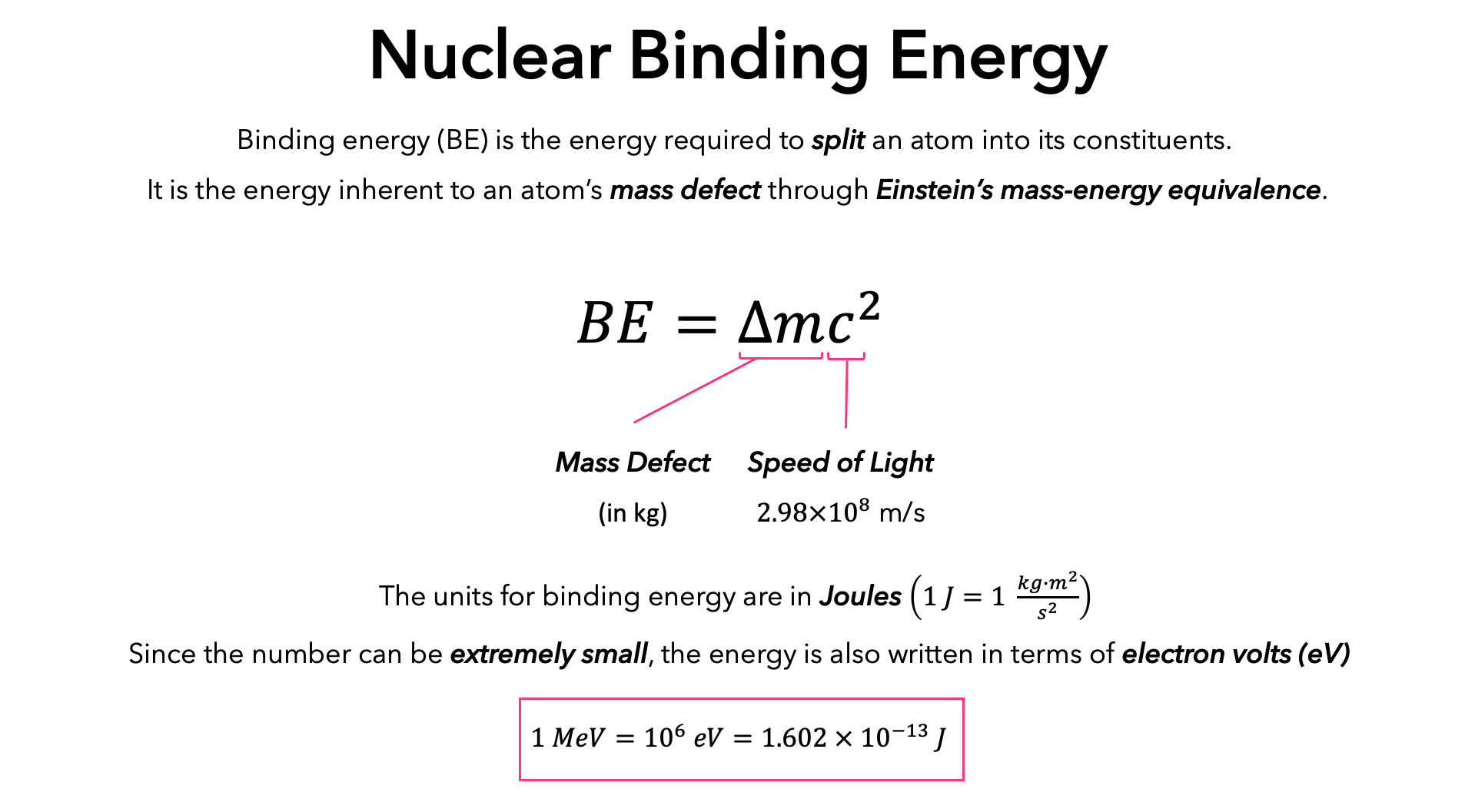
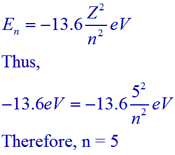
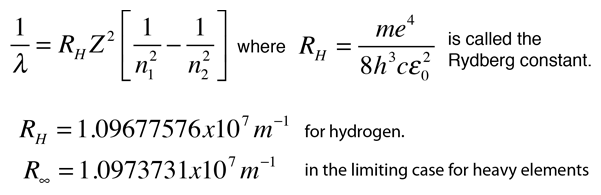
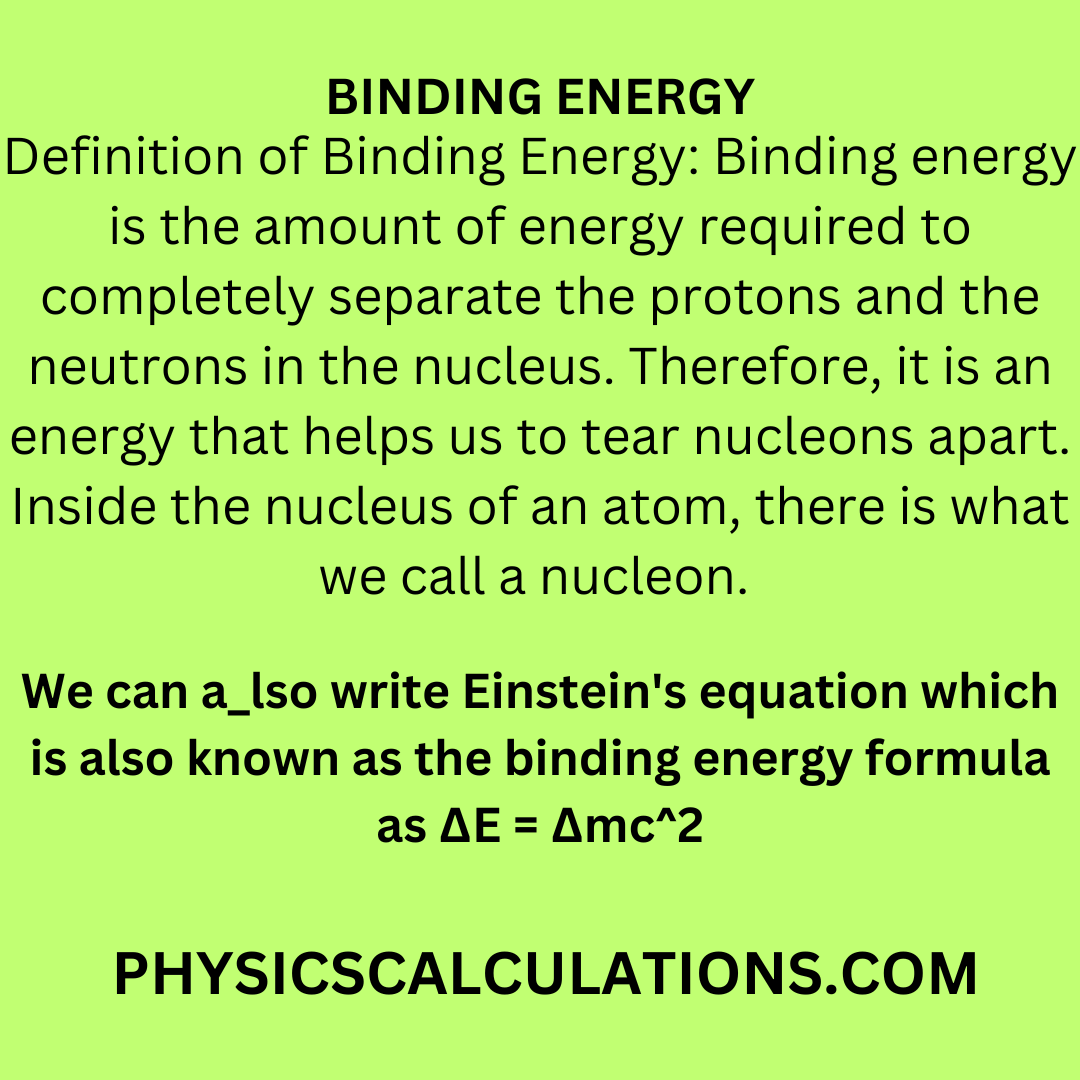



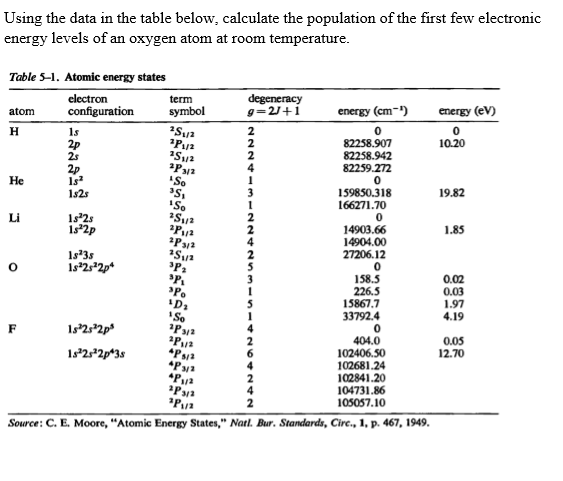

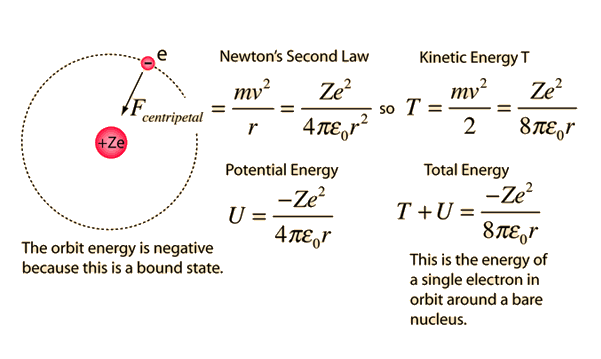
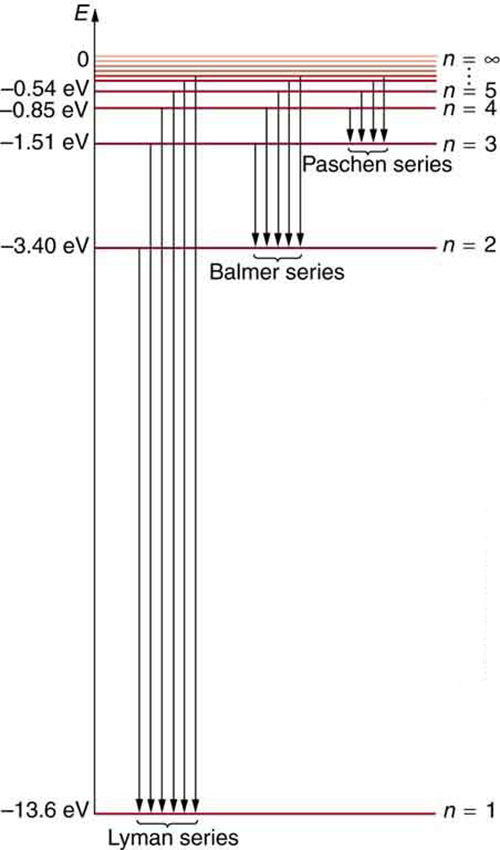
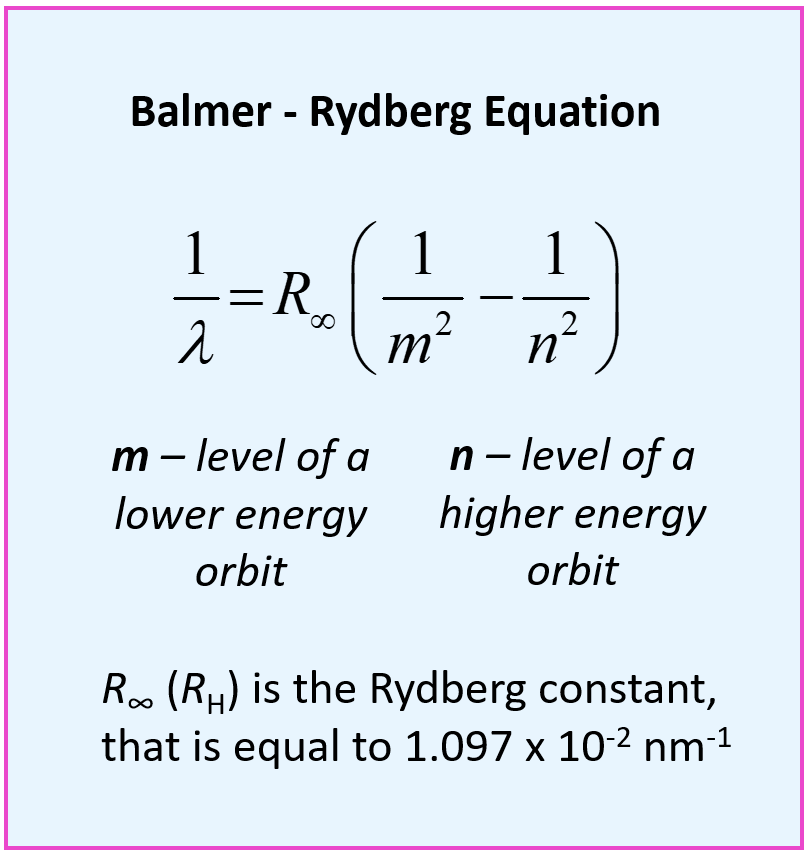
.PNG)